New Advisory Brings the Alcohol-Cancer Connection to the Forefront
Alcohol and Cancer Risk
Our year was off to a sobering start with the advisory on alcohol and cancer risk issued by the Surgeon General in January. The key points, which were concerning, and for at least half of Americans, seemingly unknown until now, included:
- A direct link was reported between alcohol consumption and higher risk for cancers of the breast, colorectum, esophagus, liver, mouth, throat (pharynx), and voice box (larynx).
- Four pathways were posited: 1) As it metabolizes, alcohol breaks down into acetaldehyde, a chemical which damages DNA in multiple ways. This can cause a cell to begin growing uncontrollably and create a cancerous tumor. 2) Alcohol induces oxidative stress, damaging DNA, proteins, and cells, and increasing inflammation. 3) Alcohol may alter hormone levels, which can play a role in development of breast cancer. 4) Carcinogens from other sources, especially particles of tobacco smoke, can dissolve in alcohol, making it easier to be absorbed into the body, and increasing risk for mouth and throat cancers.
- Citing a 2019 survey showing that just 45% of Americans recognize alcohol as a risk factor for cancer, a strong recommendation was made to raise awareness by updating the current warning label on alcoholic beverages.
There is no doubt that the Surgeon General can greatly influence recognition of a health issue, as evidenced by the landmark 1964 report on the risks of tobacco, laying the groundwork for public regulations in the decades to follow. Then, 42% of Americans smoked, now just 11% do. The impact of the current alcohol advisory may not be as profound, but it is significant for several reasons.
While the science behind the advisory is not new, as alcohol-related cancer risk has been noted previously by many healthcare organizations, it has grown increasingly stronger over the years. Particularly for women who drink, recent studies have added to the mounting evidence of their higher risk of developing breast cancer, as well as increased susceptibility to liver disease, cardiovascular disease, and neurotoxicity compared to males. A more widespread public campaign with prominent warnings on alcohol-containing products will help underscore these findings.
The advisory may also add impetus to the cultural shift around alcohol use that’s been occurring over the last decade. As noted, the percentage of Americans who agreed that 1-2 drinks per day is bad for one’s health is low at 45% in the most recent Gallup poll but still reflects a marked improvement compared to 26% in 2016. Most promisingly, the younger generation is leading the charge, with 65% of adults aged 18 to 34 agreeing that alcohol consumption negatively affects health vs. 38% on average of their elders.
Highlighting the risk of disease associated with alcohol requires a more nuanced approach than tobacco, where no consumption is considered safe. Instead, alcohol consumption is viewed along a continuum where risk for most people remains relatively low at 2 standard drinks or less per week, and moderately low at 3 to 6 standard drinks per week – yet fully acknowledging that individual risk is determined by a complex interaction of biological and environmental factors. As long-time researchers wrote in the Harvard Public Health Journal, while heavy drinking is indisputably harmful at every age, the data may not justify sweeping statements about the effects of moderate alcohol consumption. They point to research with mixed results e.g. studies showing decreased mortality, lower risk of cardiovascular disease in moderate drinkers, and a widely cited UK study of one million women that reported higher rates of breast cancer among drinkers but lower rates of thyroid cancer, non-Hodgkin lymphoma, and renal cell carcinoma. Recognizing that a gold-standard randomized control study would be well-nigh impossible to conduct, the most reasonable approach is to equip people with information that allows them to understand why less alcohol is better, and zero risk is only possible at zero consumption.
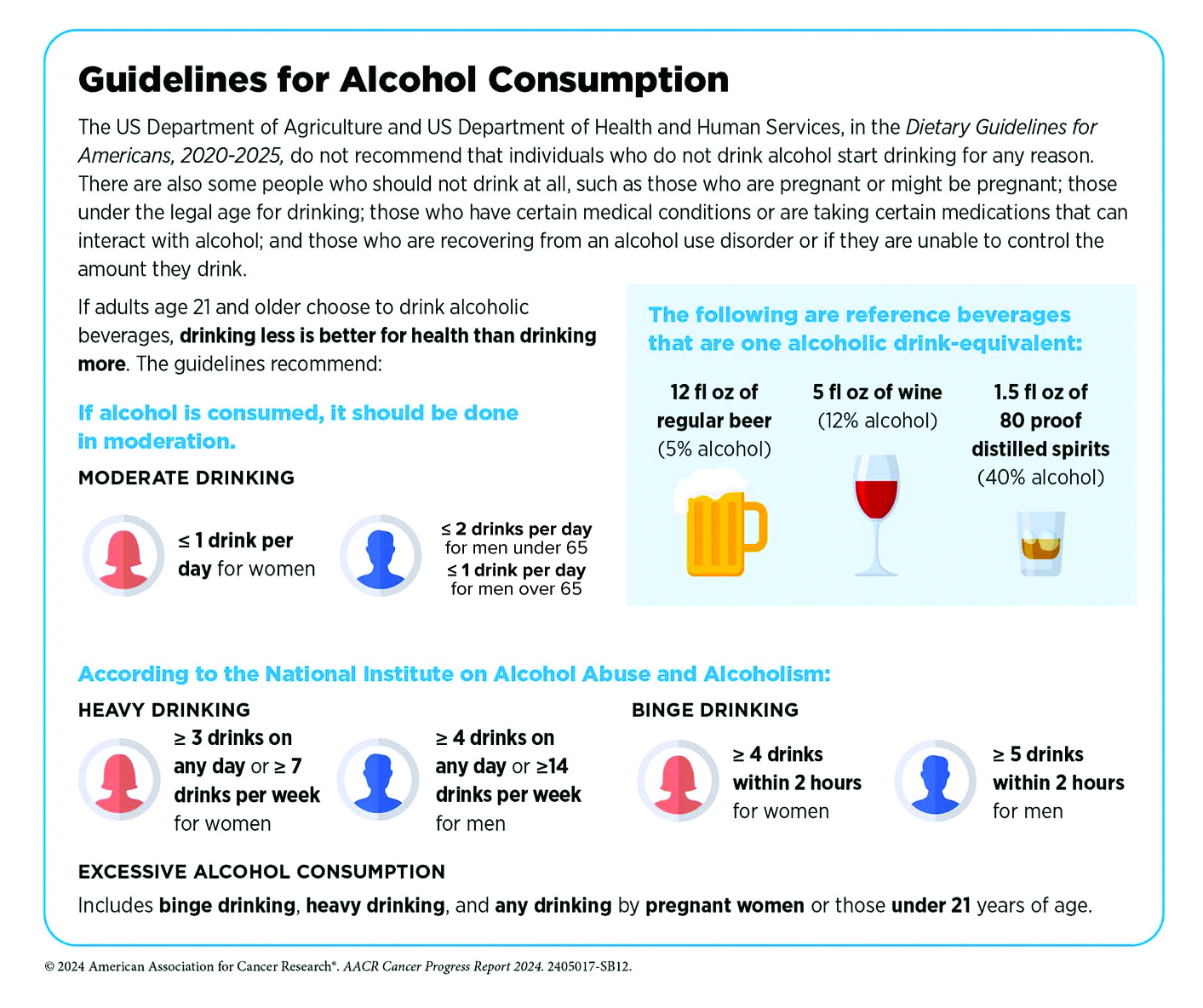
Our recommendations:
If you don’t drink, don’t start, as benefits are unproven, and the downsides are clear. Avoid alcohol completely if you are pregnant or trying, have a personal or family history of alcohol use disorder (AUD), have a medical condition that alcohol can aggravate (e.g., liver disease, precancerous conditions of the digestive tract), use medication that can cause interactions, or operate potentially dangerous machinery. However, if you are debating whether you can enjoy an occasional glass of chardonnay, please talk to us. We’ll help you make an informed decision based on multiple factors, including age, gender, medical history, diet, fitness, and lifestyle.
Symptoms of Alcohol Use Disorder (AUD)
What defines a heavy drinker, or a person with AUD? If you’ve experienced two or more of these symptoms in the past year, you may benefit from professional guidance to help you decrease or stop alcohol consumption:
- Had times when you ended up drinking more, or longer, than you intended?
- More than once wanted to cut down or stop drinking, or tried to, but couldn’t?
- Spent a lot of time being sick from drinking, or getting over other aftereffects?
- Wanted a drink so badly you couldn’t think of anything else?
- Found that drinking—or being sick from drinking—often interfered with taking care of your home or family, or caused job troubles?
- Continued to drink even though it was causing trouble with your family or friends?
- Given up or cut back on activities important to you to drink?
- Gotten into unsafe situations while or after drinking e.g., driving, walking in a dangerous area?
- Continued to drink even though it was making you feel depressed/anxious or after an alcohol-related memory blackout?
- Found that your usual number of drinks had much less effect than before?
- Experienced withdrawal symptoms when the effects of alcohol were wearing off e.g., trouble sleeping, shakiness, restlessness, nausea, sweating?
Sources: Harvard Public Health, Journal of Internal Medicine, Journal of the National Cancer Institute, Surgeon General’s Advisory on Alcohol and Cancer Risk, UptoDate, Rethinking Drinking, National Institutes of Health